Objectives: The main aim of the project is to develop a method for the identification of the TMS intensity and coil position needed for the effective stimulation of a given brain region. First, the hand area will be considered; it can be seen as a benchmark area because it is well studied in the literature. Based on the results of the hand area, the leg area will be then considered, being not as studied as the hand area, but belonging to the motor cortex for which a feedback in terms of motor evoked potential is possible. The proposed method aims at being the more general as possible, starting from brain areas easy to study as those considered in this project (areas in the motor cortex). The rationale is to transfer, in the future, the knowledge acquired in this research project to other brain areas, more difficult to stimulate. In particular, we refer to the prefrontal cortex, the different somatosensorial areas of the cortex, the cortex involved in the language and swallowing functions, or the associative areas of the cortex, that can be stimulated for treating severe depression as well as many other psychiatric and neurological disorders. For these areas a feedback of the stimulation can come only after many TMS sessions and time-consuming clinical protocols. This knowledge transfer will be developed in other research projects, which we aim to submit in the European context in the forthcoming years.
Methodologies: In this project, in order to identify the optimal coil position and TMS intensity, a cutting-edge approach is proposed. We will use personalized data (from MRI images) and consider the variability of tissue properties (in terms of Uncertainty Quantification), we will solve the forward field problem very fast by applying a Model Order Reduction (MOR) technique and finally we will solve the inverse problem in an accurate and challenging way thanks to DL techniques. Our method will be validated by means of TMS measurements. To this end, the project will be developed as follows. At the beginning of the project, 25 healthy, right-handed subjects, in the range 20-60 years will be recruited. The MRI scan of each volunteer will be acquired. T1- and T2-weighted MRIs will be performed with a 3 T scanner (Siemens Skyra), voxel size 1x1x1 mm.
The first motor threshold measurements for the hand region will be then acquired. The TMS will be delivered with a figure-of-eight coil, because of its focality with respect to the circular coil, characterized by a monophasic current. A neuronavigation system will be used during the TMS procedures. In this project, the DICOM system of coordinates (the origin is at the scanner origin, which is the center of the gradient coil, x increases from right to left, y increases from anterior to posterior, z increases from inferior to superior) will be used, so, because a neuronavigator has to be bought, a neuronavigator able to work with this system of coordinates will be considered. However, if a neuronavigation system working with the Montreal Neurological Institute (MNI) coordinates will be used for practical reasons, we plan to transform the coordinates from DICOM to MNI and vice versa by means of suitable matrices.
In this first phase of experiments, both the resting motor threshold (RMT) and the active motor threshold (AMT) will be measured by recording a Motor Evoked Potential (MEP) from a hand muscle (abductor pollicis brevis, ABP). The RMT is the lowest stimulus intensity required to elicit a MEP of at least 50μV in 5 out of 10 consecutive trials. Similarly, AMT is defined as the lowest stimulus intensity to elicit a MEP of at least 200μV in 5 out of 10 consecutive trials during an isometric contraction of ~10–20% of the maximum contraction in the target muscle.
The TMS experiments will give us the TMS intensity to elicit a MEP from the ABP muscle for each subject, which, coupled with the MRI scans, will be used for implementing the personalized field models. The MRI images will be completely anonymized and then copied on a mass storage device (e.g. CDs) following the procedures required by the ITT office of the Mondino Institute and transferred by hand to the POLIMI Unit. Starting from MRI images of a subject, the geometrical domain will be built by means of a segmentation procedure, made with the open source software SimNIBS [M1]. A Finite-Element Model (FEM) will be then implemented in SimNIBS, considering both head domain and coil geometry.
The eddy current problem will be solved with FEM coupled with Polynomial Chaos Expansion, able to consider the uncertainty in the knowledge of electrical conductivities [M2].
As a matter of fact, brain tissue characteristics vary among people, and this produces a relevant uncertainty in the results of the computation of the induced electric field in TMS. In particular, the most critical parameters are electrical conductivities, since biological tissues behave like air from the magnetic point of view. Tissue properties will be obtained from suitable databases, yet including specific uncertainty ranges to consider the high variability of parameters among patients and during the single patient lifetime [G2]. To this end, we will consider the literature data, e.g., findings from Gabriel and Gabriel [M3].
Moreover, starting from FEM and in order to obtain fast solutions of the forward eddy current problem, a Model Order Reduction (MOR) approach will be then applied [M4].
The MOR model will enable to simulate the coil in different positions around the target brain area with different orientations (overall 6 degrees of freedom, 3 for the position and 3 for the orientation are considered), with low computational costs. The obtained field maps (i.e. mean and standard deviation of the induced electric field in x-, y- and z-direction) with associated relevant positions and orientations of the coil can be collected in a database; moreover, a fast model for field simulation which considers tissue uncertainties and real head geometry will be available.
Moreover, the experiments performed by neurologists in the first phase of the project will be simulated with the help of FE models and, hence, the field map obtained during the experimental session will be computed. In this way, the field values which enable the stimulation of the cortical area of a given subject will be known.
The inverse problem can be formulated as follows: given the geometry of the head and the material properties (with their uncertainty ranges), find the optimal source position and intensity to obtain a given electric field map in a target brain area. In order to have a benchmark solution, we will solve this inverse problem with a classical optimization approach: we will use a genetic algorithm well known in literature e.g. NSGA-II [M5] coupled with the MOR field model implemented in the previous phase of the project.
A key point will be that to properly define the wanted electric field map in the target area. Many papers devoted to the identification of the induced electric field in the hand region (also called hand knob) have been published in the past years. The most recent ones identified the electric field normal to the interface between white and grey matter as the most probable cause of neuronal stimulation. In particular, for the hand region, the pre-central cortical gyrus and the anterior wall of the central sulcus are considered as target area and the preferred direction for the induced electric field is that perpendicular to the central sulcus [M6]. To solve the inverse problem, a suitable formulation has to be defined: the aim is to maximize the induced electric field (normal component) in the target areas and minimize the electric field in the regions around, which should not be stimulated. We think that a multi-objective optimization problem arises, and the NSGA-II method could be able to solve it in a very efficient way. In order to solve the same inverse problem in a new and efficient way, we plan to make use of DL [D1, D2]. With this approach we will work with images of the electric field maps.
The proposed approach is characterized by the implementation of a cascade of two Deep Neural Networks (DNNs): a Variational Autoencoder (VA) [D3, D4] and a Convolutional Neural Network (CNN) [D2, D5] (see Fig. 1). This model will be denoted as DNN model.
Starting from an image of the desired electric field map highlighted by the neurologist (we call it 'raw image'), the VA will be able to generate a FEM-like electric field map, which will be used as input for the CNN. Then, the CNN will return the coil position for obtaining the desired electric field map.

Fig. 1 - Online use of the DL approach
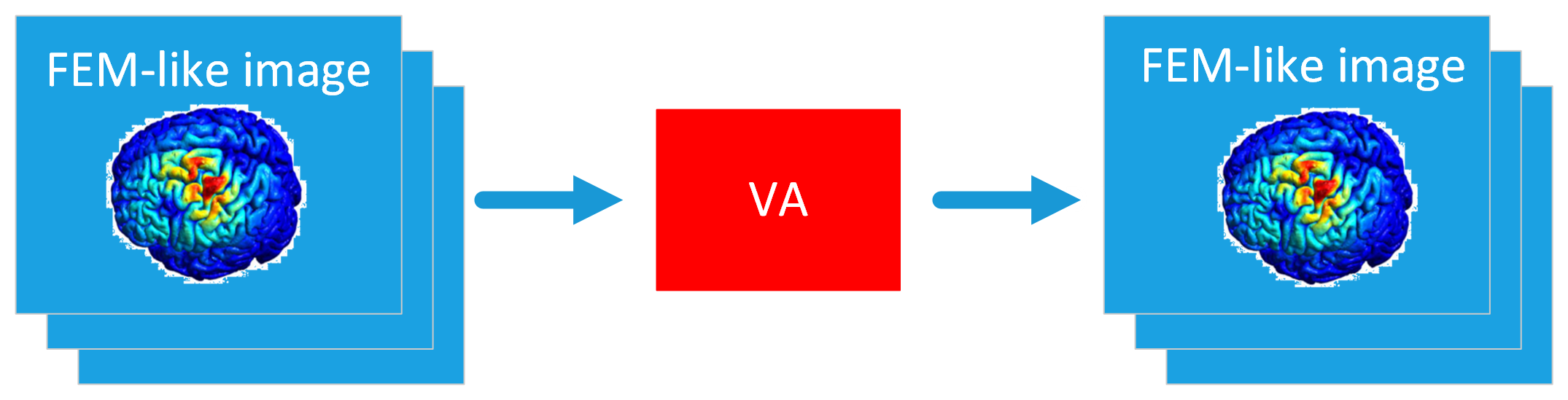
Fig. 2 - VA training
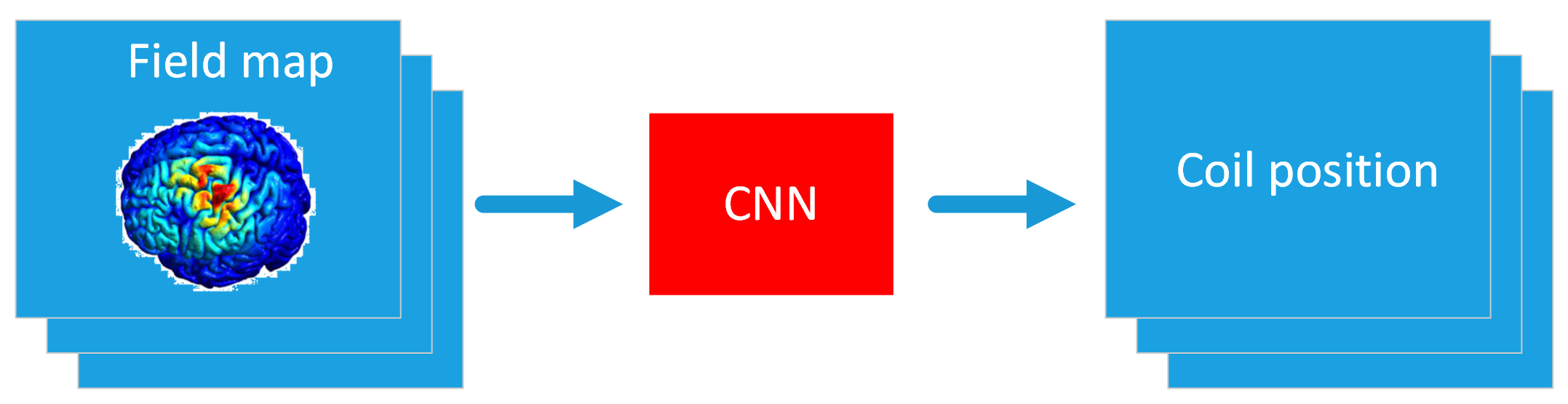
Fig. 3 - CNN training